 Suffers from Fecal Incontinence*
Suffers from Fecal Incontinence*
Fecal incontinence (FI), the involuntary passage of stool, is mainly due to internal anal sphincter (IAS) dysfunction. Current treatments do not successfully treat the underlying pathology of the disease and demonstrate disappointing long term effects.
*National Institute of Health (NIH)
Cellf BIO is actively recruiting for a Phase 1 Clinical Trial.Learn More
It's time to rejuvenate your quality of life with BioSphincter™
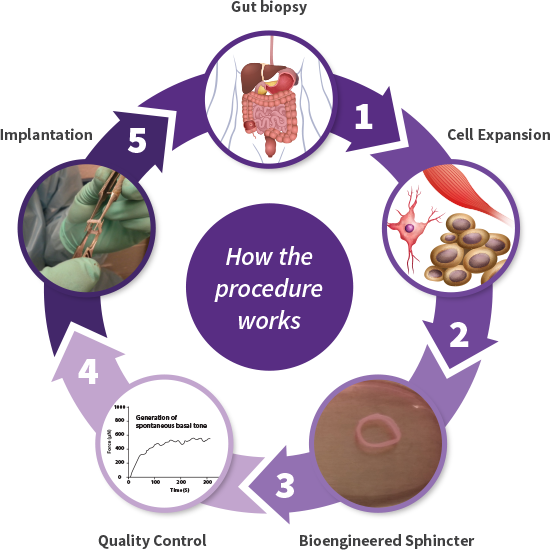
Cellf BIO manufactures bioengineered sphincters using autologous progenitor cells that replace the dysfunctional internal anal sphincter, the root cause of FI. This new product, called a BioSphincter, allows the patient to regain full control of bowel movements and rejuvenate their quality of life.
Innovative Cell-Based Technology
Cellf BIO bioengineers the BioSphincter from smooth muscle cells and neural stem cells, retrieved in a biopsy procedure, from the patient’s own gut, eliminating any risk of rejection. The newly grown autologous sphincter will be implanted in the area of the defective sphincter and will form a new continuum of the patient’s own gastro intestinal tract.
Cellf BIO maintains patent protection for the critical platform technologies and processes. These technologies also enable Cellf BIO to create additional products for urinary incontinence and esophageal reconstruction.
Addressing an Unmet Need
Fecal incontinence (FI) is the inability to control your bowel movements, causing stool (feces) to leak unexpectedly from the rectum. It is a problem faced by about 1 in 12 people, or almost 18 million adults in the US. Although FI can occur with people of any age, it is most common in older adults and women after child birth. FI affects all aspects of peoples’ lives, greatly reducing quality of physical and mental health – causing anger, frustration, depression, social anxiety, loss of confidence and self-esteem, and isolation.
Currently, there is no effective and permanent solution to the problem. Cellf BIO provides the only permanent solution that successfully restores the normal physiological function of the ano-rectum, allowing the patient to regain control of their bowel movements. The impact of restoration of bowel control will offer tremendous hope for the psychological stress, social stigma, decreased self-esteem, and decreased productivity associated with this devastating disorder.
Sale of the BioSphincter will commence commercially upon the Cellf BIO’s completion of clinical trials and obtaining regulatory approval.
